Abstract
Background: Allogeneic hematopoietic stem cell transplantation (SCT) is a highly effective consolidation strategy in acute myeloid leukemia (AML). Non-myeloablative and reduced-intensity (NMA/RIC) conditioning regimens have expanded the use of SCT to older patients (pts) (age ≥ 60 years). Age alone no longer appears to be a barrier for successful SCT, with reported 3-year overall survival (OS) rates in the 45-50% range (Maakaron 2022).
Methods: We conducted a retrospective study spanning 10 years to evaluate SCT-related outcomes in older AML. All pts ≥ 60 years presenting to our institution with newly-diagnosed (ND) AML were included. Acute promyelocytic leukemia (APL) was excluded. Median OS and relapse-free survival (RFS) were calculated using the Kaplan Meier method. A landmark analysis was performed to evaluate time-to-event endpoints in pts who underwent SCT in first response compared to those who did not.
Results: Between 7/19/2012 and 11/17/2021, we identified 1073 pts ≥ 60 years with ND-AML. The median age at diagnosis was 71 years (range 60-94), 622 (58%) were male, 189 (18%) had prior myelodysplastic/myeloproliferative neoplasm, and 220 (21%) had therapy-related AML. ELN 2017 risk was favorable in 215 (20%), intermediate in 234 (22%), and adverse in 538 (50%) pts (86 with data missing). Most pts (84%) were treated with low-intensity (LOW) regimens. Only 16% were treated with intensive (INT) chemotherapy (intermediate or higher dose cytarabine). Venetoclax (VEN) was included in 34% of the regimens. The CR/CRi rate was 60% in the overall cohort. The CR/CRi rate was 74%, 48%, and 72% in pts treated with INT, LOW, and LOW + VEN therapies, respectively. The median OS and RFS were 11.2 and 10.6 months for the full cohort, respectively.
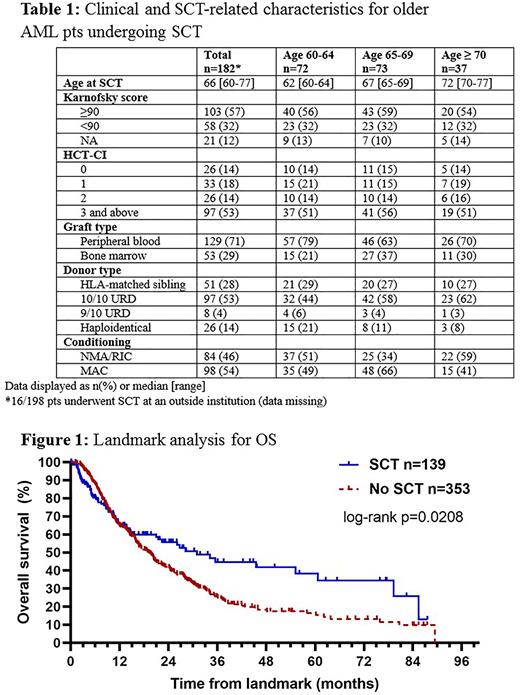
The rate of referral to the SCT service was 38% (413/1073) and increased over time (31% in 2012-2013, 52% by 2020-2021). This occurred in parallel to increasing rates of CR/CRi (53% in 2012-2013, 66% by 2020-2021), likely reflecting increased efficacy of VEN-containing regimens in the later years. We identified 198/1073 pts (18%) who underwent SCT at some point during therapy, 152/198 (77%) in first response (CR/CRi/MLFS) after frontline therapy, and 46/198 (23%) following one or more salvage regimens. Of all ND-AML pts, the SCT rate was 37%, 10%, and 22% in pts treated with INT, LOW, and LOW + VEN, respectively. The rate of undergoing SCT increased over time (15% in 2012-2013, 27% by 2020-2021). Clinical/SCT characteristics for the pts who underwent SCT are shown in table 1. The rate of grade 3/4 acute graft-vs-host disease (GVHD) was 11% and 19% of pts experienced chronic GVHD of any grade. Following SCT, 24% of pts experienced relapse of AML and 21% died without disease relapse. The 100-day mortality following SCT was 13%.
We then performed a landmark analysis (using median time to SCT [4.3 months] as the landmark) comparing pts undergoing SCT in first response following frontline therapy at our institution (n=139) to responding pts who did not receive SCT in first response and who were alive and without relapse at the landmark (n=353). Both the median OS (31.0 vs 18.9 months, p=0.0208, figure 1) and median RFS (23.2 vs 10.0 months, p<0.0001) were superior in the pts who underwent SCT compared to those who did not. Pts undergoing SCT in first response were younger (median age 66 vs 72 yrs) and had better performance status (PS 2-4 in 10% vs 22%) compared to no-SCT pts. Distribution of ELN risk categories and best response (CR/CRi/MLFS) were similar in the SCT and no-SCT groups.
In pts who responded to initial therapy and were referred for SCT, but ultimately did not undergo SCT in first response (n=161), the most common documented reasons for forgoing SCT were social reasons (pt preference, financial/insurance/caregiver issues) in 29%, disease relapse in 29%, being deemed unfit due to comorbidities in 22%, active uncontrolled infections in 11%, and other reasons in 11%. An adequate donor was documented in 66% of these pts.
Conclusions: Our results support the use of SCT as a consolidative strategy in older AML pts. Pts who undergo SCT have superior OS and RFS compared to those who do not. However, fewer than 20% of pts undergo the procedure mostly due to inadequate disease control, comorbidities, and a variety of social reasons. Recent trends indicate higher rates of SCT referral and completion as more effective anti-leukemic therapies are allowing a greater proportion of older AML pts to undergo SCT.
Disclosures
Kantarjian:KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Research Funding; ImmunoGen: Research Funding; Novartis: Honoraria, Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Takeda: Honoraria. Popat:Bayer: Research Funding; Incyte: Research Funding; Novartis: Research Funding; Abbvie: Research Funding. DiNardo:GenMab: Membership on an entity's Board of Directors or advisory committees; Forma: Research Funding; Cleave: Research Funding; Takeda: Honoraria; Novartis: Honoraria; ImmuneOnc: Honoraria, Research Funding; LOXO: Research Funding; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; Foghorn: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Bluebird Bio: Honoraria; Astellas: Honoraria; Servier: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria; Gilead: Honoraria. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Short:Novartis: Consultancy; Stemline Therapeutics: Research Funding; Astellas: Research Funding; Takeda Oncology: Consultancy, Research Funding; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; AstraZeneca: Consultancy. Jabbour:AbbVie: Other: Advisory Role, Research Funding; Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Takeda: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Genentech: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding. Konopleva:Novartis: Patents & Royalties, Research Funding; Sanofi: Other: grant support, Research Funding; Rafael Pharmaceutical: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Agios: Other: grant support, Research Funding; Ablynx: Other: Grant support, Research Funding; Calithera: Other: Grant Support, Research Funding; Cellectis: Consultancy, Other: Grant support, Research Funding; Eli Lilly: Consultancy, Patents & Royalties, Research Funding; Reata Pharmaceuticals: Current equity holder in private company, Patents & Royalties; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kisoji: Consultancy, Honoraria; Amgen: Consultancy; Forty-Seven: Consultancy, Honoraria, Other: Grant support; Stemline Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; F. Hoffman La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grant support, Research Funding; Genentech: Consultancy, Other: grant support, Research Funding; AbbVie: Consultancy, Other: grant support, Research Funding. Garcia-Manero:Aprea: Honoraria; Curis: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Astex: Consultancy, Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Gilead Sciences: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Acceleron Pharma: Consultancy. Alousi:Genetech: Consultancy; Sanofi / Kadmon: Honoraria; Prolacta: Consultancy; Mallinkrodt: Honoraria; Incyte: Honoraria, Research Funding. Shpall:Bayer: Honoraria; Affimed: Other: License agreement; Takeda: Patents & Royalties; Navan: Consultancy; Fibroblasts and FibroBiologics: Consultancy; NY blood center: Consultancy; adaptimmune: Consultancy; axio: Consultancy. Champlin:Actinium: Consultancy; General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Kadmon: Consultancy; Johnson &Johnson: Consultancy; Omeros: Consultancy; Cell Source Inc.: Research Funding. Borthakur:Catamaran Bio, Abbvie, PPD Development, Protagonist Therapeutics, Janssen: Consultancy; Astex Pharmaceuticals, Ryvu, PTC Therapeutics: Research Funding; Pacylex, Novartis, Cytomx, Bio Ascend: Membership on an entity's Board of Directors or advisory committees. Ravandi:Syos: Consultancy, Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Xencor: Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy; Biomea Fusion, Inc.: Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Amgen: Honoraria, Research Funding; Prelude: Research Funding; Abbvie: Consultancy, Honoraria, Research Funding. Kadia:JAZZ: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; Servier: Consultancy; cellenkos: Research Funding; Ascentage: Research Funding; Genfleet: Research Funding; PinotBio: Consultancy; Regeneron: Research Funding; Delta-Fly: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; AstraZeneca: Research Funding; Astellas: Research Funding; BMS: Consultancy, Research Funding; Agios: Consultancy; Novartis: Consultancy; Pfizer: Research Funding; Astex: Honoraria; Iterion: Research Funding; Glycomimetics: Research Funding; Abbvie: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal